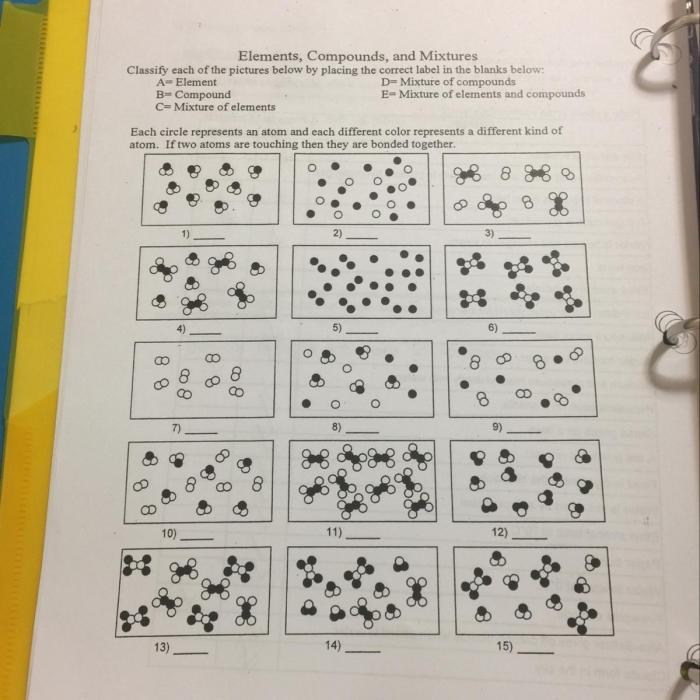
Delving into the realm of chemistry, we present the Element Compound and Mixture Worksheet Answers, an authoritative resource meticulously crafted to illuminate the fundamental concepts of elements, compounds, and mixtures. This comprehensive guide unravels their intricate definitions, properties, and distinctions, empowering learners with a profound understanding of these essential building blocks of matter.
As we embark on this scientific expedition, we will delve into the unique characteristics of elements, the intricate compositions of compounds, and the diverse nature of mixtures. Through a series of engaging examples and insightful explanations, we aim to clarify the often-confusing differences between these three fundamental categories, providing a solid foundation for further exploration in chemistry.
Elements, Compounds, and Mixtures
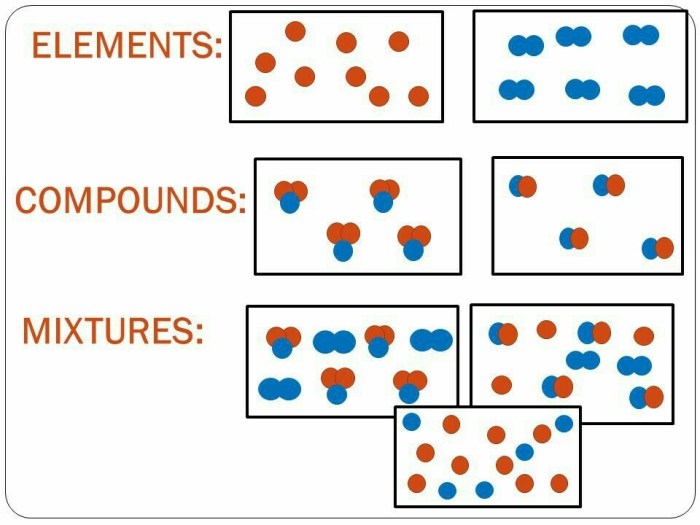
Matter can be classified into three categories: elements, compounds, and mixtures. Elements are the basic building blocks of matter, while compounds are formed when two or more elements combine chemically. Mixtures are combinations of two or more elements or compounds that are not chemically combined.
Elements
- Definition: An element is a substance that cannot be broken down into simpler substances by chemical means.
- Examples: Hydrogen, oxygen, gold, silver, carbon
- Properties: Elements are pure substances with unique chemical properties. They are the simplest form of matter and cannot be separated into simpler substances by physical means.
Compounds, Element compound and mixture worksheet answers
- Definition: A compound is a substance formed when two or more elements combine chemically in a fixed ratio.
- Examples: Water (H 2O), carbon dioxide (CO 2), sodium chloride (NaCl)
- Properties: Compounds are pure substances with unique chemical properties. They are more complex than elements and can be broken down into their constituent elements by chemical means.
Mixtures
- Definition: A mixture is a combination of two or more elements or compounds that are not chemically combined.
- Examples: Salt water, air, granite
- Properties: Mixtures are not pure substances and do not have unique chemical properties. They can be separated into their constituent elements or compounds by physical means, such as filtration or distillation.
Comparison of Elements, Compounds, and Mixtures
| Property | Element | Compound | Mixture |
|---|---|---|---|
| Definition | Cannot be broken down into simpler substances by chemical means | Formed when two or more elements combine chemically in a fixed ratio | Combination of two or more elements or compounds that are not chemically combined |
| Examples | Hydrogen, oxygen, gold | Water, carbon dioxide, sodium chloride | Salt water, air, granite |
| Properties | Pure substances with unique chemical properties | Pure substances with unique chemical properties | Not pure substances, no unique chemical properties |
FAQ Insights: Element Compound And Mixture Worksheet Answers
What is the difference between an element and a compound?
An element is a pure substance composed of only one type of atom, while a compound is a pure substance composed of two or more different types of atoms chemically bonded together.
What is the difference between a compound and a mixture?
A compound is a pure substance with a definite composition and properties, while a mixture is a combination of two or more pure substances that retain their individual properties.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.